Team 10 - PETRY
Research Goal
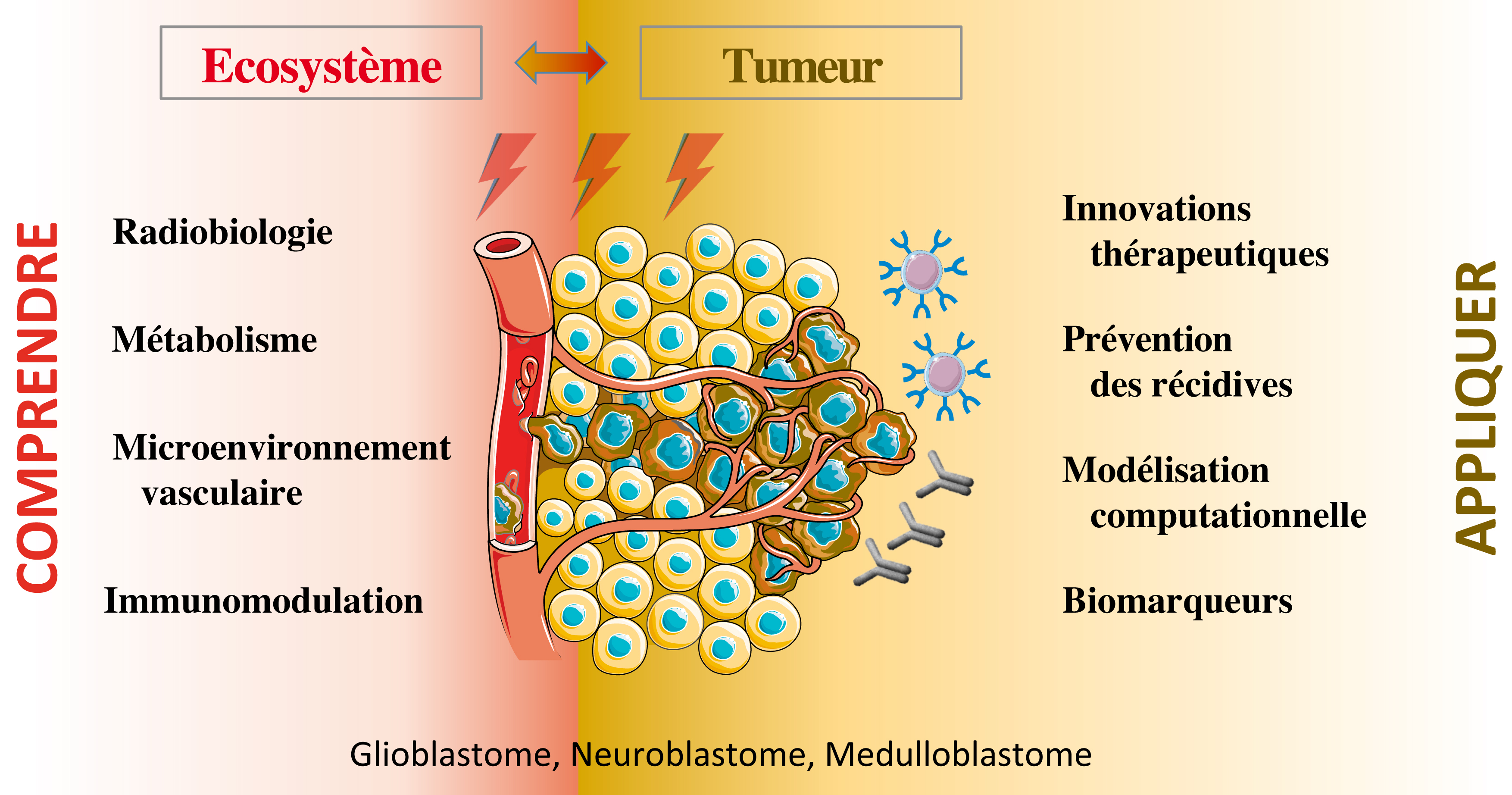
The classical therapeutic scheme for neural cancers, such as Glioblastoma and Neuroblastoma, involved surgery followed with a combination of radio- and chemotherapies. However tumor location and acquired treatment resistance limit the efficacy of these treatments. Novel non-genotoxic therapeutic approaches in combination with actual treatments are currently being explored to improve their efficacy.
On this basis, the PETRY team explores key parameters involved in tumor aggressiveness and relapse, in particular tumor metabolic adaptation and tumor microenvironment cooption.
Our research program is organized around three main axis :
1) The tumor plasticity by deciphering the dynamic evolution of multiple tumor cell states, including molecular subtypes, tumor-stem cells and metabolic heterogeneity
2) The vascular microenvironment and its impact on tumor agressivity and relapse, in particular following radiotherapy/chemotherapy
3) The development of innovative immunotherapeutic strategies through antibody and T-cell engineering
Contact us
François Paris, DR INSERM
francois.paris@univ-nantes.fr
Claire Pecqueur, DR CNRS
claire.pecqueur@univ-nantes.fr
Medias
Claire Pecqueur, DR CNRS
Sophie Fougeray, MCU
Catherine Gratas, MCU-PH
Colin Niaudet, CRCN INSERM
Lisa Oliver, Biologiste CHU
Christophe Olivier, MCU
François Vallette, DR INSERM
Noémie Joalland, Post-doctorante
Marine Devinat, IE CDD
Laetitia Durand, AI INSERM
Mylene Guérif, Tech CDD
Aline Perrin, Tech ICO
Hortense Alliot,, Doctorante
Hala Awada, Doctorante
Julianne Céroni, Doctorante
Mélanie Laurent--Blond, Doctorante
Pierre Paris, Doctorant
Estrella Passerat de la Chapelle, Doctorante
Chercheurs associés
Dr. S. Faraj, PU-CHU
Dr. A. Lisbona, PH ICO
Dr. E. Rio, PH ICOt
Dr. C. Salaud, MCU-PH
Dr. E. Thébaud, PH ICO
Main Publications :
2024 :
- Renoult O, Laurent-Blond M, Awada H, Oliver L, Joalland N, Croyal M, Paris F, Gratas C, Pecqueur C.Metabolic profiling of glioblastoma stem cells reveals pyruvate carboxylase as a critical survival factor and potential therapeutic target. Neuro Oncol. 2024 Sep 5;26(9):1572-1586. doi: 10.1093/neuonc/noae106
- Pauline Thomas, Pierre Paris, Claire Pecqueur. Arming Vd2 T cells with chimeric receptor to combat cancer. Clin. Can. Res. 2024 May 15.Online ahead of print. DOI: 10.1158/1078-0432.CCR-23-3495
- Pauline Thomas, Maëva Veerasamy, Marine Devinat, Elodie Guiet, Jocelyn Ollier, Pierre Paris, Natacha Entz-Werlé, Catherine Gratas, Béatrice Clémenceau, Stéphane Birklé, François Paris, Claire Pecqueur, Sophie Fougeray. Targeting pediatric High-Grade Gliomas with OAcGD2-CAR Vδ2 T cells. BIORXIV. doi: https://doi.org/10.1101/2023.11.17.567375.
2023 :
- Awada H, Paris F, Pecqueur C. Exploiting radiation immunostimulatory effects to improve glioblastoma outcome. Neuro Oncol. 2023 Mar 14;25(3):433-446. doi: 10.1093/neuonc/noac239. PMID: 36239313; PMCID: PMC10013704.
- Niaudet C, Jung B, Kuo A, Swendeman S, Bull E, Seno T, Crocker R, Fu Z, Smith LEH, Hla T. Therapeutic activation of endothelial sphingosine-1-phosphate receptor 1 by chaperone-bound S1P suppresses proliferative retinal neovascularization. EMBO Mol Med. 2023 May 8;15(5):e16645. doi: 10.15252/emmm.202216645. Epub 2023 Mar 13. PMID: 36912000 Free PMC article.
- Rafia C, Loizeau C, Renoult O, Harly C, Pecqueur C, Joalland N, Scotet E. The antitumor activity of human Vγ9Vδ2 T cells is impaired by TGF-β through significant phenotype, transcriptomic and metabolic changes. Front Immunol. 2023 Jan 19;13:1066336. doi: 10.3389/fimmu.2022.1066336. PMID: 36741364; PMCID: PMC9893774.
- Pu Y, Li L, Peng H, Liu L, Heymann D, Robert C, Vallette F, Shen S. Drug-tolerant persister cells in cancer: the cutting edges and future directions. Nat Rev Clin Oncol. 2023 Nov;20(11):799-813. doi: 10.1038/s41571-023-00815-5. Epub 2023 Sep 25. PMID: 37749382 Review.
2022 :
- Ophélie Renoult, Catherine Gratas, Noémie Joalland, Mélanie Laurent--Blond, Hala Awada, Marine Bourgeois, Lisa Oliver, Sophie Chiavassa, Mikaël Croyal, François Paris, Claire Pecqueur. Pyruvate carboxylation identifies Glioblastoma Stem-like Cells opening new metabolic strategy to prevent tumor recurrence. BIORXIV. doi:https://doi.org/10.1101/2022.07.18.500427
- Alzial G, Renoult O, Paris F, Gratas C, Clavreul A, Pecqueur C. Wild-type isocitrate dehydrogenase under the spotlight in glioblastoma. Oncogene. 2022 Jan;41(5):613-621. doi: 10.1038/s41388-021-02056-1. Epub 2021 Nov 11. PMID: 34764443; PMCID: PMC8799461.
- Charlotte Degorre, Ophélie Renoult, Ann Christin Parplys, Hala Awada, Anne Clavreul, Manon Pietri, Lisa Oliver, Noemie Joalland, Michelle Ricoul, Catherine Gratas, François Vallette, Kirsten Borgmann, Laure Sabatier, Claire Pecqueur*, François Paris*. Mechanistic insights of radiation-induced endothelial senescence impelling glioblastoma genomic instability at relapse. BioRXIV doi: https://doi.org/10.1101/2021.12.13.472364.
- Rabé M, Fonteneau L, Oliver L, Morales-Molina A, Jubelin C, Garcia-Castro J, Heymann D, Gratas C, Vallette FM. Cellular Heterogeneity and Cooperativity in Glioma Persister Cells Under Temozolomide Treatment. Front Cell Dev Biol. 2022 May 25;10:835273. doi: 10.3389/fcell.2022.835273. eCollection 2022. PMID: 35693929 Free PMC article.
- Garnier D, Ratcliffe E, Briand J, Cartron PF, Oliver L, Vallette FM. The Activation of Mesenchymal Stem Cells by Glioblastoma Microvesicles Alters Their Exosomal Secretion of miR-100-5p, miR-9-5p and let-7d-5p. Biomedicines. 2022 Jan 6;10(1):112. doi: 10.3390/biomedicines10010112. PMID: 35052791 Free PMC article.
- Lalier L, Vallette F, Manon S. Bcl-2 Family Members and the Mitochondrial Import Machineries: The Roads to Death. Biomolecules. 2022 Jan 19;12(2):162. doi: 10.3390/biom12020162. PMID: 35204663 Free PMC article. Review.
- Jubelin C, Muñoz-Garcia J, Griscom L, Cochonneau D, Ollivier E, Heymann MF, Vallette FM, Oliver L, Heymann D. Three-dimensional in vitro culture models in oncology research. Cell Biosci. 2022 Sep 11;12(1):155. doi: 10.1186/s13578-022-00887-3. PMID: 36089610 Free PMC article. Review.
2021 :
- Thomas P, Galopin N, Bonérandi E, Clémenceau B, Fougeray S, Birklé S. CAR T Cell Therapy's Potential for Pediatric Brain Tumors Cancers (Basel). 2021 Oct 29;13(21):5445. doi: 10.3390/cancers13215445. PMID: 34771608 Free PMC article. Review.
- Alzial G, Renoult O, Paris F, Gratas C, Clavreul A, Pecqueur C. Wild-type isocitrate dehydrogenase under the spotlight in glioblastoma. Oncogene. 2022 Jan;41(5):613-621. doi: 10.1038/s41388-021-02056-1. Epub 2021 Nov 11. PMID: 34764443 Free PMC article. Review.
- Lalier L, Mignard V, Joalland MP, Lanoé D, Cartron PF, Manon S, Vallette FM. TOM20-mediated transfer of Bcl2 from ER to MAM and mitochondria upon induction of apoptosis. Cell Death Dis. 2021 Feb 15;12(2):182. doi: 10.1038/s41419-021-03471-8. PMID: 33589622 Free PMC article.
- Poczobutt JM, Mikosz AM, Poirier C, Beatman EL, Serban KA, Gally F, Cao D, McCubbrey AL, Cornell CF, Schweitzer KS, Berdyshev EV, Bronova IA, Paris F, Petrache I. Altered Macrophage Function Associated with Crystalline Lung Inflammation in Acid Sphingomyelinase Deficiency. Am J Respir Cell Mol Biol. 2021 May;64(5):629-640. doi: 10.1165/rcmb.2020-0229OC. PMID: 33662226 Free PMC article
- Almeida L, Estrada-Rodriguez G, Oliver L, Peurichard D, Poulain A, Vallette F. Treatment-induced shrinking of tumour aggregates: a nonlinear volume-filling chemotactic approach J Math Biol. 2021 Aug 24;83(3):29. doi: 10.1007/s00285-021-01642-x. PMID: 34427771
- Prasanna PG, Citrin DE, Hildesheim J, Ahmed MM, Venkatachalam S, Riscuta G, Xi D, Zheng G, Deursen JV, Goronzy J, Kron SJ, Anscher MS, Sharpless NE, Campisi J, Brown SL, Niedernhofer LJ, O'Loghlen A, Georgakilas AG, Paris F, Gius D, Gewirtz DA, Schmitt CA, Abazeed ME, Kirkland JL, Richmond A, Romesser PB, Lowe SW, Gil J, Mendonca MS, Burma S, Zhou D, Coleman CN. Therapy-Induced Senescence: Opportunities to Improve Anticancer Therapy. J Natl Cancer Inst. 2021 Oct 1;113(10):1285-1298. doi: 10.1093/jnci/djab064. PMID: 33792717 Free PMC article.
- Foucault A, Ravalet N, Besombes J, Picou F, Gallay N, Babin L, Bourgeais J, Hamard S, Domenech J, Loyer P, Vallet N, Lejeune J, Gyan E, Béné MC, Vallette F, Olivier C, Hérault O. Low-Dose Pesticides Alter Primary Human Bone Marrow Mesenchymal Stem/Stromal Cells through ALDH2 Inhibition. Cancers (Basel). 2021 Nov 14;13(22):5699. doi: 10.3390/cancers13225699. PMID: 34830855 Free PMC article.
- Olivier C, Oliver L, Lalier L, Vallette FM. Drug Resistance in Glioblastoma: The Two Faces of Oxidative Stress. Front Mol Biosci. 2021 Jan 27;7:620677. doi: 10.3389/fmolb.2020.620677. eCollection 2020. PMID: 33585565 Free PMC article. Review.
- Bahri M, Kailayangiri S, Vermeulen S, Galopin N, Rossig C, Paris F, Fougeray S, Birklé S. SIRPα-specific monoclonal antibody enables antibody-dependent phagocytosis of neuroblastoma cells. Cancer Immunol Immunother. 2022 Jan;71(1):71-83. doi: 10.1007/s00262-021-02968-7. Epub 2021 May 22. PMID: 34023958
2020 :
- Garnier D, Renoult O, Alves-Guerra MC, Paris F, Pecqueur C. Glioblastoma Stem-Like Cells, Metabolic Strategy to Kill a Challenging Target. Front Oncol. 2019. 9:118.
- Fleurence J, Bahri M, Fougeray S, Faraj S, Vermeulen S, Pinault E, Geraldo F, Oliver O, Véziers J, Marquet P, Rabé M, Gratas C, Vallette F, Pecqueur C, Paris F, Birklé S. Impairing Temozolomide Resistance Driven by Glioma Stem-Like Cells With Adjuvant Immunotherapy Targeting O-acetyl GD2 Ganglioside. Int. J. Cancer. 2020. 146 (2): 424-438
- Rabé M, Dumont S, Álvarez-Arenas A, Janati H, Belmonte-Beitia J, Calvo GF, Thibault-Carpentier C, Séry Q, Chauvin C, Joalland N, Briand F, Blandin S, Scotet E, Pecqueur C, Clairambault J, Oliver L, Perez-Garcia V, Nadaradjane A, Cartron PF, Gratas C, Vallette FM. Identification of a transient state during the acquisition of temozolomide resistance in glioblastoma. Cell Death Dis. 2020. 11(1):19
- Pecqueur C & F. Paris. When mitochondrial metabolism meets tumour cell radiobiology. ESTRO Newsletter Sept 2020
- Oizel K, Yang C, Renoult O, Gautier F, Do QN, Joalland N, Gao X, Ko B, Vallette F, Ge WP, Paris F, DeBerardinis RJ, Pecqueur C. Glutamine uptake and utilization of human mesenchymal Glioblastoma in orthotopic mouse model. Cancer & Metabolism 2020; 8: 9. Published online 2020 Aug 10.
Ketteler J, Leonetti D, Veas Roy V, Estephan H, Wittka A, Maier P, Reis H, Herskind C, Jendrossek V, Paris F, Klein D. Caveolin 1 regulates the ASMase/ ceramide-mediated radiation-response of endothelial cells in the context of tumor-stroma-interactions. Cell Death Dis. 2020. Apr; 11(4): 228 - Leonetti D, Estéphan H, Ripoche N, Dubois N, Aguesse A, Gouard S, Brossard L, Chiavassa S, Corre I, Pecqueur C, Neunlist M, Hadchity E, Gaugler MH, Mahé MM, Paris F. Secretion of acid sphingomyelinase and ceramide by endothelial cells contributes to radiation-induced intestinal toxicity. 2020. Cancer Res. 2020 Jun 15;80(12):2651-2662. doi: 10.1158/0008-5472.CAN-19-1527. Epub 2020 Apr 14.
- Averbeck D, Candéias S, Chandna S , Foray N, Friedl AA, Haghdoost S, Jeggo PA, Lumniczky K, Paris F, Quintens R, Sabatier L. Establishing Mechanisms Affecting the Individual Response to Ionizing Radiation. Int J Radiat Biol. 2020. 1:1-27
- Oliver L, Lalier L, Salaud C, Heymann D, Cartron PF, Vallette FM. Drug resistance in glioblastoma: are persisters the key to therapy? Cancer Drug Resist 2020 Aug 7;3(3):287-301: DOI: 10.20517/cdr.2020.29
- Duforestel M, Briand J, Bougras-Cartron G, Heymann D, Frenel JS, Vallette FM, Cartron PF. Cell-free circulating epimarks in cancer monitoring. Epigenomics. 2020 Jan 9. doi: 10.2217/epi-2019-0170
- Potiron V, Clément-Colmou K, Jouglar E, Pietri M, Chiavassa S, Delpon G, Paris F, Supiot S. Tumor Vasculature Remodeling by Radiation Therapy Increases Doxorubicin Distribution and Efficacy. Cancer Lett. 2020. 457: 1-9
- Clément-Colmou K, Potiron V, Pietri M, Guillonneau M, Jouglar E, Chiavassa S, Delpon G, Paris F, Stéphane Supiot. Influence of Radiotherapy Fractionation Schedule on the Tumor Vascular Microenvironment in Prostate and Lung Cancer Models. Cancers. 2020 12 (1).
Dufresne S, Guéritat J, Chiavassa S, Noblet C, Assi M, Rioux-Leclercq N, Rannou-Bekono F, Lefeuvre-Orfila L, Paris F, Rébillard A. Exercise training improves radiotherapy efficiency in a murine model of prostate cancer. FASEB J 2020. Accepted. PMID 32043634 - Mignard V, Dubois N, Lanoé D, Joalland MP, Oliver L, Pecqueur C, Heymann D, Paris F, Vallette FM, Lalier L. Sphingolipids Distribution at Mitochondria-Associated Membranes (MAM) Upon Induction of Apoptosis. 2020. J Lipid Res. 2020 Jul;61(7):1025-1037.
- Salaud C, Alvarez-Arenas A, Geraldo F, Belmonte-Beitia J, Calvo GF, Gratas C, Pecqueur C, Garnier D, Pérez-Garcià V, Vallette FM, Oliver L. Mitochondria transfer from tumor-activated stromal cells (TASC) to primary Glioblastoma cells. Biochem Biophys Res Commun. 2020 Sep 14:S0006-291X(20)31699-5. doi: 10.1016/j.bbrc.2020.08.101.
- Briand J, Garnier D, Nadaradjane A, Clément-Colmou K, Potiron V, Supiot S, Bougras-Cartron G, Frenel JS, Heymann D, Vallette FM, Cartron PF. Radiotherapy-induced overexpression of exosomal miRNA-378a-3p in cancer cells limits natural killer cells cytotoxicity. Epigenomics. 2020 Mar;12(5):397-408. doi: 10.2217/epi-2019-0193
- Cheray M, Etcheverry A, Jacques C, Pacaud R, Bougras-Cartron G, Aubry M, Denoual F, Peterlongo P, Nadaradjane A, Briand J, Akcha F, Heymann D, Vallette FM, Mosser J, Ory B, Cartron PF. Cytosine methylation of mature microRNAs inhibits their functions and is associated with poor prognosis in glioblastoma multiforme. Mol Cancer. 2020 Feb 25;19(1):36. doi: 10.1186/s12943-020-01155-z
- Briand J, Sérandour AA, Nadaradjane A, Bougras-Cartron G, Heymann D, Ory B, Vallette FM, Cartron PF. N6-Adenosine Methylation of miRNA-200b-3p Influences Its Functionality and Is a Theranostic Tool. Mol Ther Nucleic Acids. 2020 Aug 14;22:72-83. doi: 10.1016/j.omtn.2020.08.010
- Pérez-García VM, Calvo GF, Bosque JJ, León-Triana O, Jiménez J, Pérez-Beteta J, Belmonte-Beitia J, Valiente M, Zhu L, García-Gómez P, Sánchez-Gómez P, Hernández-San Miguel E, Hortigüela R, Azimzade Y, Molina-García D, Martínez A, Acosta Rojas A, Ortiz de Mendivil A, Vallette FM, Schucht P, Albillo D, Pérez-Cano M, Murek M, Honguero Martínez AF, Jiménez Londoño GA, Arana E, García Vicente AM. Universal scaling laws rule explosive growth in human cancers. Nat Phys. 2020 Dec;16(12):1232-1237. doi: 10.1038/s41567-020-0978-6. Epub 2020 Aug 10. PMID: 33329756 Free PMC article.
- Pariset E, Penninckx S, Kerbaul CD, Guiet E, Macha AL, Cekanaviciute E, Snijders AM, Mao JH, Paris F, Costes SV. 53BP1 Repair Kinetics for Prediction of In Vivo Radiation Susceptibility in 15 Mouse Strains. Radiat Res. 2020 Nov 10;194(5):485-499. doi: 10.1667/RADE-20-00122.1 PMID: 32991727
- Guyon N, Garnier D, Briand J, Nadaradjane A, Bougras-Cartron G, Raimbourg J, Campone M, Heymann D, Vallette FM, Frenel JS, Cartron PF Anti-PD1 therapy induces lymphocyte-derived exosomal miRNA-4315 release inhibiting Bim-mediated apoptosis of tumor cells. Cell Death Dis. 2020 Dec 11;11(12):1048. doi: 10.1038/s41419-020-03224-z. PMID: 33311449
2019 :
- Leveque X, Hochane M, Geraldo F, Dumont S, Gratas C, Oliver L, Gaignier C, Trichet V, Layrolle P, Heymann D, Herault O, Vallette FM, Olivier C. Low-Dose Pesticide Mixture Induces Accelerated Mesenchymal Stem Cell Aging In Vitro. Stem Cells. 2019. 37(8):1083-1094.
- Supiot S, Rousseau C, Dore M, Chèze-Le-Rest C, Kandel-Aznar C, Potiron V, Guerif S, Paris F, Ferrer L, Campion L, Meingan P, Delpon G, Hatt M, Visvikis D. Reoxygenation during radiotherapy in intermediate-risk prostate cancer. Radiother Oncol. 2019.133:16-19.
- Sosa Marrero C, Acosta O, Castro M, Hernandez, Rioux-Leclercq N, Paris F, de Crevoisier R. Sensitivity analysis of an in silico model of tumor growth and radiation response. IEEE. 2019. Processing article. ISBI.
- Briand J, Nadaradjane A, Bougras-Cartron G, Olivier C, Vallette FM, Cartron PF. Diuron exposure and Akt overexpression promote glioma formation through DNA hypomethylation. Clinical Epigenetics. 2019
- Duforestel M, Nadaradjane A, Bougras-Cartron G, Olivier C, Frenel JS, Vallette FM, Lelièvre S, Cartron PF. Glyphosate primes mammary cells for tumorigenesis by reprogramming the epigenome in a TET3-dependent manner. Frontiers in Genetics. 2019
- Valès S, Bacola G, Biraud M, Touvron M, Bessard A, Geraldo F, Dougherty KA, Lashani S, Bossard C, Flamant M, Duchalais E, Marionneau-Lambot S, Oullier T, Oliver L, Neunlist M, Vallette FM, Van Landeghem L. Tumor cells hijack enteric glia to activate colon cancer stem cells and stimulate tumorigenesis. EBioMedicine. 2019 Nov;49:172-188. doi: 10.1016/j.ebiom.2019.09.045. Epub 2019 Oct 26.
- Ginisty A, Oliver L, Arnault P, Vallette F, Benzakour O, Coronas V.. The vitamin K-dependent factor, protein S, regulates brain neural stem cell migration and phagocytic activities towards glioma cells. Eur J Pharmacol. 2019 Jul 15;855:30-39. doi: 10.1016/j.ejphar.2019.04.039. Epub 2019 Apr 24
- Chauvin C, Joalland N, Perroteau J, Jarry U, Lafrance L, Willem C, Retière C, Oliver L, Gratas C, Gautreau-Rolland L, Saulquin X, Vallette FM, Vié H, Scotet E, Pecqueur C. NKG2D Controls Natural Reactivity of Vγ9Vδ2 T Lymphocytes against Mesenchymal Glioblastoma Cells. Clin Cancer Res. 2019. 25(23):7218-7228
- Aguilar E, Esteves P, Sancerni T, Lenoir V, Aparicio T, Bouillaud F, Dentin R, Prip-Buus C, Ricquier D, Pecqueur C, Guilmeau S, Alves-Guerra MC. UCP2 Deficiency Increases Colon Tumorigenesis by Promoting Lipid Synthesis and Depleting NADPH for Antioxidant Defenses. Cell Rep. 2019. 28(9):2306-2316
- Bahri M, Fleurence J, Faraj S, Ben Mostefa Daho M, Fougeray S, Birklé S. Potentiation of Anticancer Antibody Efficacy by Antineoplastic Drugs: Detection of Antibody-drug Synergism Using the Combination Index Equation. J Vis Exp. 2019;(143).
- Marin E, Bouchet-Delbos L, Renoult O, Louvet C, Nerriere-Daguin V, Managh AJ, Even A, Giraud M, Vu Manh TP, Aguesse A, Bériou G, Chiffoleau E, Alliot-Licht B, Prieur X, Croyal M, Hutchinson JA, Obermajer N, Geissler EK, Vanhove B, Blancho G, Dalod M, Josien R, Pecqueur C, Cuturi MC, Moreau A. Human Tolerogenic Dendritic Cells Regulate Immune Responses through Lactate Synthesis. Cell Metab. 2019. 30(6):1075-1090
2018 :
- Kilens S, Meistermann D, Moreno D, Chariau C, Gaignerie A, Reignier A, Lelièvre Y, Casanova M, Vallot C, Nedellec S, Flippe L, Firmin J, Song J, Charpentier E, Lammers J, Donnart A, Marec N, Deb W, Bihouée A, Le Caignec C, Pecqueur C, Redon R, Barrière P, Bourdon J, Pasque V, Soumillon M, Mikkelsen TS, Rougeulle C, Fréour T, David L Parallel derivation of isogenic human primed and naive induced pluripotent stem cells. Nat Commun. 2018. 9(1):360
- Jarry U, Joalland N, Chauvin C, Clemenceau B, Pecqueur C, Scotet E. Stereotactic Adoptive Transfer of Cytotoxic Immune Cells in Murine Models of Orthotopic Human Glioblastoma Multiforme Xenografts. J Vis Exp. 2018. (139):57870
- Joalland N, Chauvin C, Oliver L, Vallette FM, Pecqueur C, Jarry U, Scotet E IL-21 Increases the Reactivity of Allogeneic Human Vγ9Vδ2 T Cells Against Primary Glioblastoma Tumors. J Immunother. 2018. 41(5):224-231
- Brown HK, Tellez-Gabriel M, Cartron PF, Vallette FM, Heymann MF, Heymann D. Characterization of circulating tumor cells as a reflection of the tumor heterogeneity: myth or reality? Drug Discov Today. 2019 Mar;24(3):763-772. doi: 10.1016/j.drudis.2018.11.017. Epub 2018 Nov 26. Review.
- Noblet C, Delpon G, Supiot S, Potiron V, Paris F, Chiavassa S. A new tissue segmentation method to calculate 3D dose in small animal radiation therapy. Radiat Oncol. 2018. 13(1):32
- Vallette FM, Olivier C, Lézot F, Oliver L, Cochonneau D, Lalier L, Cartron PF, Heymann D. Dormant, quiescent, tolerant and persister cells: Four synonyms for the same target in cancer.Biochem Pharmacol. 2019 Apr;162:169-176. doi: 10.1016/j.bcp.2018.11.004. Epub 2018 Nov 9. Review.
- Nadaradjane A, Briand J, Bougras-Cartron G, Disdero V, Vallette FM, Frenel JS, Cartron PF. miR-370-3p Is a Therapeutic Tool in Anti-glioblastoma Therapy but Is Not an Intratumoral or Cell-free Circulating Biomarker. Mol Ther Nucleic Acids. 2018 Dec 7;13:642-650. PMID: 30497054.
- Briand J, Nadaradjane A, Vallette FM, Cartron PF (2018) The TET2 Expression Level Correlates with a Short Relapse Time in Glioblastoma Multiforme. J Clin Epigenet 4:12. doi: 10.21767/2472-1158.100097
- Chalopin A, Tellez-Gabriel M, Brown HK, Vallette F, Heymann MF, Gouin F, Heymann D.. Isolation of circulating tumor cells in a preclinical model of osteosarcoma: Effect of chemotherapy. J Bone Oncol. 2018 Jul 26;12:83-90. doi: 10.1016/j.jbo.2018.07.002. eCollection 2018 Sep
- Supiot S, Rousseau C, Dore M, Cheze-Le-Rest C, Kandel-Aznar C, Potiron V, Guerif S, Paris F, Ferrer L, Campion L, Meingan P, Delpon G, Hatt M, Visvikis D. Evaluation of tumor hypoxia prior to radiotherapy in intermediate-risk prostate cancer using 18F-fluoromisonidazole PET/CT: a pilot study. Oncotarget. 2018. 9(11):10005-10015.
- Guipaud O, Jaillet C, Clément-Colmou K, François A, Supiot S, Milliat F. The importance of the vascular endothelial barrier in the immune-inflammatory response induced by radiotherapy. British Journal of Radiology (2018)
2017 :
- Oizel K, Chauvin C, Oliver L, Gratas C, Geraldo F, Jarry U, Scotet E, Rabe M, Alves-Guerra MC, Teusan R, Gautier F, Loussouarn D, Compan V, Martinou JC, Vallette FM, Pecqueur C. Efficient Mitochondrial Glutamine Targeting Prevails Over Glioblastoma Metabolic Plasticity. Clin Cancer Res. 2017. 23(20):6292-6304
- Fleurence J, Fougeray S, Bahri M, Cochonneau D, Clémenceau B, Paris F, Heczey A, Birklé S. Targeting O-Acetyl-GD2 Ganglioside for Cancer Immunotherapy. J Immunol Res. 2017; 2017. 5604891.
- Niaudet C, Bonnaud S, Guillonneau M, Gouard S, Gaugler MH, Dutoit S, Ripoche N, Dubois N, Trichet V, Corre I, Paris F. Plasma membrane reorganization links acid sphingomyelinase /ceramide to p38 MAPK pathways in endothelial cells apoptosis. Cell. Signal. 2017. ;33:10-21
- Lafargue A, Degorre C, Corre I, Alves-Guerra MC, Gaugler MH, Vallette F, Pecqueur C, Paris F. Ionizing radiation induces long-term senescence in endothelial cells through mitochondrial respiratory complex II dysfunction and superoxide generation. Free Radic Biol Med. 2017. 108:750-759.
- Corre I, Paris F, Houle F, Huot J. p38, a major pleiotropic pathway that transduces stress and metastatic signals in endothelial cells. Oncotarget. 2017. 8:55684-714
- Faraj S, Bahri M, Fougeray S, El Roz A, Fleurence J, Véziers J, Leclair MD, Thébaud E, Paris F, Birklé S. Neuroblastoma chemotherapy can be augmented by immunotargeting O-acetyl-GD2 tumor-associated ganglioside. Oncoimmunology. 2017. 7(1):e1373232.
- Hochane M, Trichet V, Pecqueur C, Avril P, Oliver L, Denis J, Brion R, Amiaud J, Pineau A, Naveilhan P, Heymann D, Vallette FM, Olivier C. Low-Dose Pesticide Mixture Induces Senescence in Normal Mesenchymal Stem Cells (MSC) and Promotes Tumorigenic Phenotype in Premalignant MSC. Stem Cells. 2017. 35(3):800-811
- Harford-Wright E, Andre-Gregoire G, Jacobs KA, Treps L, Le Gonidec S, Leclair HM, Gonzalez-Diest S, Roux Q, Guillonneau F, Loussouarn D, Oliver L, Vallette FM, Foufelle F, Valet P, Davenport AP, Glen RC, Bidere N, Gavard J. Pharmacological targeting of apelin impairs glioblastoma growth. Brain. 2017 Nov 1;140(11):2939-2954. doi: 10.1093/brain/awx253
- Raimbourg J, Joalland MP, Cabart M, de Plater L, Bouquet F, Savina A, Decaudin D, Bennouna J, Vallette FM, Lalier L. Sensitization of EGFR Wild-Type Non-Small Cell Lung Cancer Cells to EGFR-Tyrosine Kinase Inhibitor Erlotinib. Mol Cancer Ther. 2017 Aug;16(8):1634-1644. doi: 10.1158/1535-7163.MCT-17-0075. Epub 2017 May 18.
- Alligand B, Le Breton M, Marquis D, Vallette F, Fleury F. Functional effects of diphosphomimetic mutations at cAbl-mediated phosphorylation sites on Rad51 recombinase activity. Biochimie. 2017 Aug;139:115-124. doi: 10.1016/j.biochi.2017.05.020. Epub 2017 May 29
- Peyraga G, Lizee T, Gustin P, Clement-Colmou K, Di Bartolo C, Supiot S, Mahe MA, François S, Mege M. Treatment of cutaneous and/or soft tissue manisfestations of corticostreroids refractory graft versus host disease (cGVHD) by a total nodal irradiation (TNI). Clin Transplant. 2017 Apr;31(4)
- Clement-Colmou K, Roualdes V, Martin SA, Josset S, Desal H, Campion L, Thillays F. Dynamic conformal arc radiosurgery for arteriovenous malformations : Outcome and influence of clinical and dosimetrical data. Radiother Oncol. 2017 May;123(2):251-256
- Séry Q, Rabé M, Oliver L, Vallette FM, Gratas C. HB-EGF is associated with DNA damage and Mcl-1 turnover in human glioma cell lines treated by Temozolomide. Biochem Biophys Res Commun. 2017. 493(4):1377-1383.
- Pautu V, Leonetti D, Lepeltier E, Clere N, Passirani C. Nanomedicine as a potent strategy in melanoma tumor microenvironment. Pharmacological Research (2017)
- Paul-Gilloteaux P, Potiron V, Delpon G, Supiot S, Chiavassa S, Paris F, Costes SV. Optimizing radiotherapy protocols using computer automata to model tumor cell death in function of oxygen diffusion processes. Sci Rep. 2017. 23;7(1):2280.
- Master 2 (6 internships with Experimental Biology Profile)
1- Impact of irradiation on self-organized vascular networks
Contact : Colin Niaudet, NEXT researcher (colin.niaudet@inserm.fr)
Background: PETRY team at CNRCI²NA studies the impact of radiotherapy on brain tumors, especially glioblastomas, and the surrounding healthy tissue. In particular, we investigate the changes affecting the vascular system in brain after radiation, using mouse and 3D cellular models (bioprints, organochips).
Project: The Master II project aims at developing a self-organized, 3D vascular network and analyze the effect of ionizing radiation over its architecture and functionality. The student will use cell culture of various models including reporter-tagged cell lines, perform seeding in 3D matrices, and follow-up after irradiation using video- or confocal- microscopy.
Perspectives: Results from in vivo and 3D models will be integrated to better delineate the effects of radiation over the vascular system and help devise strategies to protect patients from radiotherapy side effects.
2- MiRNA3681HG and therapeutic resistance of glioblastoma
Contact : Cartherine Gratas, MCU-PH (catherine.gratas@univ-nantes.fr)
Long non-coding RNA (lncRNA) regulate vital biological processes, and are increasingly shown as instrumental in cancers. We have found that the lncRNA, miR3681HG, is preferentially overexpressed in a subtype of aggressive glioblastoma (GBM), the most common brain cancer in adults, and its expression might be regulated by the microenvironment. Our aim is to study the role of miR3681HG in GBM by overexpressing miR3681HG in tumor cells derived from GBM patients, and by focusing on cell proliferation, migration, and metabolism. This will be performed in 2D and 3D cultures +/- cancer associated fibroblasts derived from mesenchymal stromal cells. These cultures will be treated by irradiation+/-temozolomide (standard GBM therapy) to evaluate the role of miR3681HG in treatment resistance. We will also analyze the global effect of miR3681HG using global transcriptome sequencing. We expect to identify roles of this miR3681HG on GBM progression and/or on the response to treatments.
3- Impact of the senescent tumor microenvironment on neuroblastoma relapse
Contact : Noémie Joalland, Postdoctoral Researcher (noemie.joalland@univ-nantes.fr)
Background: Neuroblastoma is a pediatric tumor that derives from nerves and generally localizes along the abdominal sympathetic chains. It occurs around the first year of live and for children aged of 18 months and over, after a surgical resection, aggressive rounds of combined chemotherapies represent the standard treatment. Unfortunately, in about 40% of cases, tumor relapse, became chemo-resistant and metastasis in bones and bone marrow. To better understand mechanisms of resistance to standard treatment and propose new therapeutic strategies, PETRY team is interesting to the contribution of the senescent tumor microenvironment. Senescence is a natural processing of aging but it can be induced by genotoxic stress, such as radio or chemotherapy, and modify both phenotype and secretome of peritumoral microvasculature. The team already demonstrates that radiotherapy induces endothelial senescence in glioblastoma patients, leading to radio-resistance and increased aggressiveness of relapse.
Project: The Master II will participate to the description of the impact of endothelial senescence on neuroblastoma chemoresistance and metastasis capability. This project combines characterization of the secretome of senescent endothelial cells and functional assays on neuroblastoma cell lines. The final goal was to validate CXCL12/CXCR4 as a chemokine/receptor couple to target in new therapeutic strategy for neuroblastoma patient in relapse.
Techniques: cell culture, videomicroscopy, molecular biology, ELISA assay
Contact : François Paris, DR INSERM (francois.paris@inserm.fr)
Background:PETRY team in the CRCI²NA is contributing to glioblastoma research by demonstrating the importance of the vascular network in acute and chronic responses to radiotherapy. PETRY observes that radiological stress induces early aging of the peritumoral microvasculature, known as endothelial senescence, leading to increased aggressiveness of GBM relapses.
Project: The Master II project will contribute to the research and development of new anti-cancer strategies to eliminate these senescent endothelial cells. During its training, the student will specifically carry out technologies in cellular and molecular biology, confocal and live microscopy, with the support of the PETRY team and national core facilities. Precisely, transcriptomic characterization of endothelial senescence followed by a functional screening will be performed during the internship. Critical molecular targets will later be inhibited by inducible shRNAs to understand their roles in senescence.
Prospects: These results will represent a first step towards the development of dedicated pharmacological treatments enhancing the efficacy of radiotherapy by specifically targeting radiation-induced vascular dysfunctions.
5- Molecular dynamics of Glioblastoma cells.
Contact : Claire Pecqueur, DR CNRS (claire.pecqueur@univ-nantes.fr)
Glioblastoma (GBM), the most common and aggressive form of primary brain tumor in adults, has a very poor prognosis, with less than 5% survival at 5 years and systematic relapse. These highly heterogeneous tumors are composed of multiple tumor cells characterized by distinct cellular states. The most resistant to treatment, and consequently most frequently found in relapses, is the MES state (Clin. Can. Res 2017; Cancer Metab 2020; BioRXIV 2022). In collaboration with the laboratory of G. Gargiulo (Berlin), our team aims to identify the dynamic evolution of these states using innovative synthetic genetic tracers (sGT) specific to each molecular state.
In this Master 2 project, the student will study the relevance of various designed sGTs (4xMES, 2xCL, 2xPN), depending on the initial molecular signature of tumors, the microenvironment and treatments (RT, CT). The student will carry out cell biology (culture, transduction, 3D-model), molecular (WB) and phenotypic (microscopy, FACS) characterization to identify the most relevant sGTs for each state. Taken together, these data will provide robust tools for monitoring the evolution of tumor cell states, and subsequently identify a strategy to prevent MES-mediated tumor escape.
The student will be trained in all technologies, to become autonomous during his/her internship. This project is part of the team's scientific research program, to gain a better understanding in the dynamic evolution of molecular and cellular heterogeneity in glioblastoma, to propose new therapeutic avenues in the long-term (PLBIO INCA 2024-28).
Contact : Claire Pecqueur, DR CNRS (claire.pecqueur@univ-nantes.fr)
Glioblastoma is the most common and most aggressive primary brain tumor in adults, despite the extensive treatment options available. This bleak prognosis is mainly due to the systematic recurrence of this cancer. Immunotherapy is a promising new therapeutic approach for many cancers. In collaboration with CRCI2NA team 12, we have developed a strategy combining spontaneous cytotoxicity of LTVd2-CAR against tumors with CAR-mediated cytotoxicity (Clin. Can. Res 2019, BioRXIV 2023).
In this Master 2 project, the student will test the immunoreactivity of LTVd2-CAR against primary glioblastoma cultures derived from primary and recurrent tumors. To develop this project, the student will be responsible for culturing tumor cells and lymphocytes. In the 1st phase, primary cultures will be analyzed on a molecular (RNAseq), protein (markers and ligands involved in LTVd2-CAR and CAR recognition by FACS, WB and microscopy), and phenotypic (GSC, metabolism, radio and chemoresistance by LDA, Seahorse/fluxomics, and MTT) levels. Then, the student will perform degranulation (FACS) and cytotoxicity (microscopy, FACS) assays to characterize LTVd2-CAR immunoreactivities. Taken together, these data will provide strong preliminary results to determine the temporality of immunotherapy in glioblastoma at recurrence.
The student will be trained in all technologies, to become autonomous during his internship. This project is part of the team's scientific research program into the development of immunotherapy for glioblastoma (PAIR Gliome 2021-25).
- PhD Thesis
- C-NU : Lymphocytes T CAR à logique booléenne pour une éradiation efficace des Gliomes de Haut-grade
- contact : Claire Pecqueur (claire.pecqueur@univ-nantes.fr)
- C-NU : Modulation of vascular senescence to limit glioblastoma agressiveness after radiotherapy
- contact : François Paris (francois.paris@univ-nantes.fr)
- C-NU : Lymphocytes T CAR à logique booléenne pour une éradiation efficace des Gliomes de Haut-grade
- Post docs
- No specific announcement at the moment.
- Others job opportunities
- No specific announcement at the moment.
If you are interested in joining the team, please feel free to submit your spontaneous applications to us.
























