Team 1: ITMI
Immunomodulation of the Tumor Microenvironment and Immunotherapy of Thoracic Cancers
Team leaders: Dr Christophe Blanquart & Jean-François Fonteneau
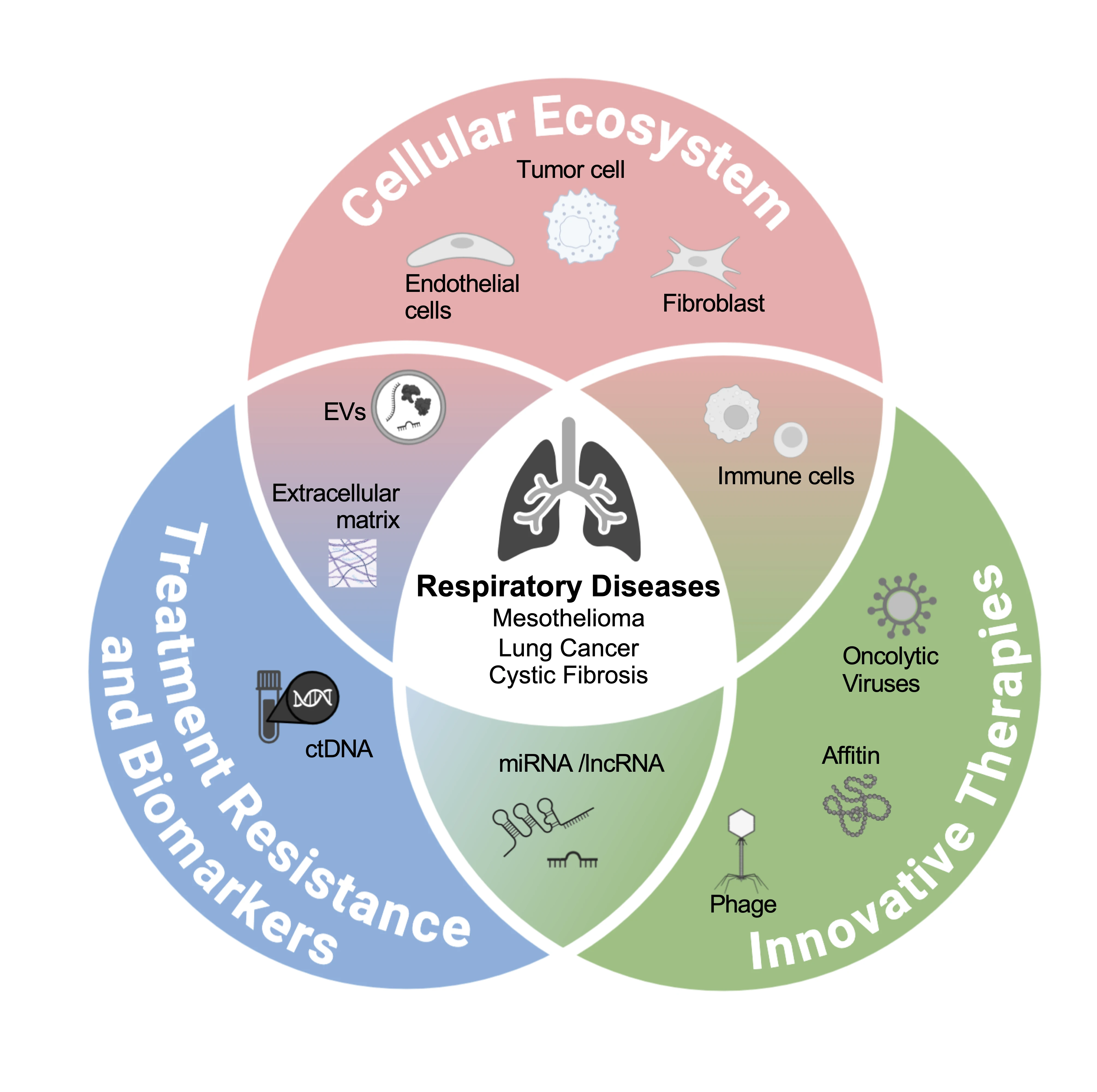
Understanding the pathological pulmonary ecosystem mainly in cancer but also in cystic fibrosis, is essential for developing more effective and innovative therapeutic strategies. In this context, our team investigates thoracic diseases through three major research axes: i) deciphering the pathological thoracic ecosystem with a particular attention to macrophages and endothelial cells; ii) characterizing treatment responses, including resistance mechanisms and the identification of robust biomarkers; and iii) designing novel anti-tumor therapeutic approaches based on immunogenic cell death induction, therapeutic proteins, and engineered oncolytic viruses.
Our research projects :
Study of the tumor-associated macrophages (TAMs) in the tumor microenvironment and development of novel therapies
Christophe Blanquart

Modeling endothelial cell’s (ECs) interactions within the tumor microenvironment
Lucas Treps

Remodeling of the endothelial compartment in lung cancer upon therapies: Mechanisms and consequences
Isabelle Corre

Non-coding RNAs in response to therapies in lung cancer (Learn more)
Delphine Fradin

Sensitivity of tumor cells to oncolytic viruses and induction of immunogenic cell death
Jean-François Fonteneau

Modulation of the tumor microenvironment with oncolytic viruses
Nicolas Boisgerault

We use a unique collection of patient-derived cell lines and biocollections of pathological and healthy specimens (pleural fluids, blood, tissues) from mesothelioma or lung cancer patients.
Team members

Christophe Blanquart, DR2 (CNRS)
Jean-François Fonteneau, CRHC (INSERM)
Nicolas Boisgerault, CRCN (INSERM)
Isabelle Corre, CRHC (CNRS)
Delphine Fradin, CRCN (INSERM)
Lucas Treps, CRCN (CNRS)
Laurent Cellerin, PH (CHU Nantes)
Anne-Laure Chéné, AHU (CHU Nantes)
Murielle Corvaisier-Chiron, CEC (CHU Nantes)
Marc Denis, PU-PH (CHU Nantes)
Chloé Sauzay, MCU-PH (CHU Nantes)
Elvire Pons-Tostivint, MCU-PH (CHU Nantes)
Christine Sagan, PH (CHU Nantes)
Tiphaine Delaunay, Post-doctorante
Saiveth Hernandez-Hernandez, Post-doctorante
Mégane Willems, Post-doctorante
Propanna Bandyopadhyay, Post-doctorante
Virginie Dehame, TR (CHU Nantes)
Sophie Deshayes, AI (CNRS)
Judith Fresquet, IE (INSERM)
Louise Gaillard, TR (INSERM)
Manon Chang, PhD Student
Emilie Navarro, PhD Student
Thomas Papazyan, PhD Student
Hortense Perdrieau, PhD Student
Manon Robert, PhD Student
About us
Selected Publications
Axis1
Krejbich M, Navarro E, Fresquet J, Cotinat M, Isen V, Perdrieau H, Forest V, Doméné A, Delaunay T, Awada H, Dochez V, Roulois D, Boisgerault N, Redon R, Blanquart C, Corre I, Treps L. In vitro models to mimic tumor endothelial cell-mediated immune cell reprogramming in lung adenocarcinoma. J Exp Clin Cancer Res. 2025 Nov 27.
Joalland N, Quemener A, Deshayes S, Humeau R, Maillasson M, LeBihan E, Salama A, Fresquet J, Mortier E*, Blanquart C*, Guillonneau C* and Anegon I*. A new soluble CSF1R-dimeric mutein with enhanced trapping of both CSF-1 and IL-34 reduces suppressive tumor-associated macrophages in mesothelioma. J Immunother Cancer. 2025. 17;13(3):e010112.
Axis2
Besse B, Pons-Tostivint E, Park K, Hartl S, Forde PM, Hochmair MJ, Awad MM, Thomas M, Goss G, Wheatley-Price P, Shepherd FA, Florescu M, Cheema P, Chu QSC, Kim SW, Morgensztern D, Johnson ML, Cousin S, Kim DW, Moskovitz MT, Vicente D, Aronson B, Hobson R, Ambrose HJ, Khosla S, Reddy A, Russell DL, Keddar MR, Conway JP, Barrett JC, Dean E, Kumar R, Dressman M, Jewsbury PJ, Iyer S, Barry ST, Cosaert J, Heymach JV. Biomarker-directed targeted therapy plus durvalumab in advanced non-small-cell lung cancer: a phase 2 umbrella trial. Nat Med. 2024 Mar;30(3):716-729. doi: 10.1038/s41591-024-02808-y.
Labbé M, Chang M, Saintpierre B, Letourneur F, de Beaurepaire L, Véziers J, Deshayes S, Cotinat M, Fonteneau JF, Blanquart C, Potiron V, Supiot S, Fradin D. Loss of miR-200c-3p promotes resistance to radiation therapy via the DNA repair pathway in prostate cancer. Cell Death Dis. 2024 Oct 16;15(10):751.
Axis3
Hirigoyen U, Guilbaud C, Krejbich M, Fouet M, Fresquet J, Arnaud B, Com E, Pineau C, Cadiou G, Burlaud-Gaillard J, Erbs P, Fradin D, Labarrière N, Fonteneau JF, Petithomme T, Boisgerault N. Oncolytic viruses alter the biogenesis of tumor extracellular vesicles and influence their immunogenicity. Mol Ther Oncol. 2024 Sep 26;32(4):200887.
Chatelain C, Berland L, Grard M, Jouand N, Fresquet J, Nader J, Hirigoyen U, Petithomme T, Combredet C, Pons-Tostivint E, Fradin D, Treps L, Blanquart C, Boisgerault N, Tangy F, Fonteneau JF. Interplay between oncolytic measles virus, macrophages and cancer cells induces a proinflammatory tumor microenvironment. Oncoimmunology. 2024 Jul 10;13(1):2377830.
Blondy T, Poly J, Linot C, Boucard J, Allard-Vannier E, Nedellec S, Hulin P, Hénoumont C, Larbanoix L, Muller RN, Laurent S, Ishow E*, Blanquart C*. Impact of RAFT chain transfer agents on the polymeric shell density of magneto-fluorescent nanoparticles and their cellular uptake. Nanoscale. 2022 Apr 14;14(15):5884-5898.
Fundings:














Partners:


- Assié JB, Meiller C, Stern E, Lasvergnas J, Arnould M, Pan L, Montagne F, Sequeiros R, Al Zreibi C, Del Nery E, Genovesio A, Lantuejoul S, Le Pimpec-Barthes F, Zucman-Rossi J, Jaurand MC, Blanquart C, Wald O, Jean D. Pharmacogenomic Characterization of a Large Cohort of Patient-Derived Cell Lines Identifies Therapeutic Strategies for Pleural Mesothelioma. Cancer Res. 2026 2;86(1):196-212. doi: 10.1158/0008-5472.CAN-24-3822.
- Chang M, Papazyan T, Pons-Tostivint E, Fradin D. Unlocking the power of non-coding RNAs: toward real-time cancer monitoring in precision oncology. Mol Cancer. 2026 Jan 9;25(1):34. doi: 10.1186/s12943-025-02536-y.
2025 :
- Briolay T*, Petithomme T*, Gravoueille H, Fresquet J, Lambot S, Cossard P, Mouratou B, Fortun A, Bernardeau K, Quéméner A, Maillasson M, Boisgerault N, Mortier E, Davodeau F, Pecorari P*, and Blanquart C*. Development of potent Affitin-based bispecific NK cell engagers for the therapy of MSLN-expressing cancers. Mol Ther Oncol. 2025. 19;33(4):201095.
- Krejbich M, Navarro E, Fresquet J, Cotinat M, Isen V, Perdrieau H, Forest V, Doméné A, Delaunay T, Awada H, Dochez V, Roulois D, Boisgerault N, Redon R, Blanquart C, Corre I, Treps L. In vitro models to mimic tumor endothelial cell-mediated immune cell reprogramming in lung adenocarcinoma. J Exp Clin Cancer Res. 2025. 27;45(1):15.
- Blanquart C and Scherpereel A. Optimizing response to immunotherapies in Pleural Mesothelioma: Clinical data and alternative models for the evaluation of new strategies. Thorax. 2025. Accepted October 2025
- Fonteneau JF, Boisgerault N, Tangy F. Antitumor immuno-virotherapy with attenuated strains of measles virus.Virologie (Montrouge). 2025 Aug 18;29(4):271-291. doi: 10.1684/vir.2025.1101.
- Costantini A, Takam Kamga P, Pons-Tostivint E, Fradin D, Emile JF, Giroux-Leprieur E. Soluble PD-L1 (sPD-L1) as a biomarker of durable response and survival in patients with advanced non-small cell lung cancer (NSCLC) treated with first-line immune checkpoint inhibitors (ICIs). Cancer Immunol Immunother. 2025 Aug 26;74(9):294. doi: 10.1007/s00262-025-04126-9.
- Lefebvre CC, Giowachini P, Derrien J, Naour M, Corre I, Thirouard L, Douillard E, Chiron D, Guillonneau F, Treps L, Campone M, Juin PP, Souazé F. MCL-1 as a molecular switch between myofibroblastic and pro-angiogenic features of breast cancer-associated fibroblasts. Cell Death Dis. 2025 Aug 9;16(1):603. doi: 10.1038/s41419-025-07920-6. PMID: 40783386.
- Joalland N, Quemener A, Deshayes S, Humeau R, Maillasson M, LeBihan E, Salama A, Fresquet J, Mortier E*, Blanquart C*, Guillonneau C* and Anegon I*. A new soluble CSF1R-dimeric mutein with enhanced trapping of both CSF-1 and IL-34 reduces suppressive tumor-associated macrophages in mesothelioma. J Immunother Cancer. 2025. 17;13(3):e010112. PMID: 40101804.
- Legrand M, Renault S, De Pinieux G, Bourreau C, Brion R, Le Nail LR, Blandin S, Hulin P, Verrecchia F, Redini F, Trichet V & Corre I. Endothelial remodeling in osteosarcoma microenvironment: Insights from patient samples and in vitro studies. Am J Cancer Res. 2025 15;15(4):1629-1646. doi: 10.62347/KYFO6159
- Clara Bourreau, Emilie Navarro, Marine Cotinat, Morgane Krejbich, Isabelle Corre, François Guillonneau, Catherine Guette, Alice Boissard, Cécile Henry, Lucas Treps, Nicolas Clere. Secretomes from non-small cell lung cancer cells induce endothelial plasticity through a partial endothelial-to-mesenchymal transition. Cancer Medecine. 2025. 14(5):e70707. doi: 10.1002/cam4.70707. PMID: 40028673.
2024 :
- Cuenot S, Fillaudeau A, Briolay T, Fresquet J, Blanquart C, Ishow E, Zykwinska A. Poroelastic and viscoelastic properties of soft materials determined from AFM force relaxation and force-distance curves. J Mech Behav Biomed Mater. 2024 Dec 9;163:106865. doi: 10.1016/j.jmbbm.2024.106865.PMID: 39662287.
- Willems M, Hamaidia M, Fontaine A, Grégoire M, Halkin L, Vilanova Mañá L, Terres R, Jamakhani M, Deshayes S, Brostaux Y, Heinen V, Louis R, Duysinx B, Jean D, Wasielewski E, Scherpereel A, Blanquart C, Willems L. The impact of Charcot-Leyden Crystal protein on mesothelioma chemotherapy: targeting eosinophils for enhanced chemosensitivity. EBioMedicine. 2024. 28;109:105418. doi: 10.1016/j.ebiom.2024.105418. PMID: 39471751.
- Labbé M, Chang M, Saintpierre B, Letourneur F, de Beaurepaire L, Véziers J, Deshayes S, Cotinat M, Fonteneau JF, Blanquart C, Potiron V, Supiot S, Fradin D. Loss of miR-200c-3p promotes resistance to radiation therapy via the DNA repair pathway in prostate cancer. Cell Death Dis. 2024. 16;15(10):751. doi: 10.1038/s41419-024-07133-3.
- Hirigoyen U, Guilbaud C, Krejbich M, Fouet M, Fresquet J, Arnaud B, Com E, Pineau C, Cadiou G, Burlaud-Gaillard J, Erbs P, Fradin D, Labarrière N, Fonteneau JF, Petithomme T, Boisgerault N. Oncolytic viruses alter the biogenesis of tumor extracellular vesicles and influence their immunogenicity. Molecular Therapy: Oncology. 2024. 32(4).
- Rossi M, Martinengo B, Diamanti E, Salerno A, Rizzardi N, Fato R, Bergamini C, Souza de Oliveira A, de Araújo Marques Ferreira T, Andrade Holanda C, Antonio Soares Romerio L, de Nazaré Correia Soeiro M, Nunes K, Ferreira de Almeida Fiuza L, Meuser Batista M, Fraga C, Alkhalaf H, Elmahallawy EK, Ebiloma G, De Koning H, Vittorio S, Vistoli G, Blanquart C, Bertrand P and Bolognesi ML. Benign-by-Design SAHA Analogues for Human and Animal Vector Borne Parasitic Diseases. ACS Med. Chem. Lett. 2024. Accepted August 8, 2024.
- Lebas M, Chinigò G, Courmont E, Bettaieb L, Machmouchi A, Goveia J, Beatovic A, Van Kerckhove J, Robil C, Angulo FS, Vedelago M, Errerd A, Treps L, Gao V, Delgado De la Herrán HC, Mayeuf-Louchart A, L'homme L, Chamlali M, Dejos C, Gouyer V, Garikipati VNS, Tomar D, Yin H, Fukui H, Vinckier S, Stolte A, Conradi LC, Infanti F, Lemonnier L, Zeisberg E, Luo Y, Lin L, Desseyn JL, Pickering J, Kishore R, Madesh M, Dombrowicz D, Perocchi F, Staels B, Pla AF, Gkika D, Cantelmo AR. Integrated single-cell RNA-seq analysis reveals mitochondrial calcium signaling as a modulator of endothelial-to-mesenchymal transition. Sci Adv. 2024 Aug 9;10(32):eadp6182.
- Declercq M, Treps L, Geldhof V, Conchinha NV, de Rooij LPMH, Subramanian A, Feyeux M, Cotinat M, Boeckx B, Vinckier S, Dupont L, Vermeulen F, Boon M, Proesmans M, Libbrecht L, Pirenne J, Monbaliu D, Jochmans I, Dewerchin M, Eelen G, Roskams T, Verleden S, Lambrechts D, Carmeliet P, Witters P. Single-cell RNA sequencing of cystic fibrosis liver disease explants reveals endothelial complement activation. Liver Int. 2024 Jun 7.
- Chatelain C, Berland L, Grard M, Jouand N, Fresquet J, Nader J, Hirigoyen U, Petithomme T, Combredet C, Pons-Tostivint E, Fradin D, Treps L, Blanquart C, Boisgerault N, Tangy F, Fonteneau JF. Interplay between oncolytic measles virus, macrophages and cancer cells induces a proinflammatory tumor microenvironment. Oncoimunol. 2024. Accepted July 2024.
- Hulo P, Deshayes S, Fresquet J, Chéné AL, Blandin S, Boisgerault N, Fonteneau JF, Treps L, Denis MG, Bennouna J, Fradin D, Pons-Tostivint E, Blanquart C. Use of non-small cell lung cancer multicellular tumor spheroids to study the impact of chemotherapy. Respir Res. 2024. 5;25(1):156.
- Briolay T, Fresquet J, Meyer D, Kerfelec B, Chames P, Ishow E, Blanquart C. Specific Targeting of Mesothelin-Expressing Malignant Cells Using Nanobody-Functionalized Magneto-Fluorescent Nanoassemblies. Int J Nanomed. 2024. 20;19:633-650
2023:
- Bessonneau-Gaborit V, Cruard J, Guerin-Charbonnel C, Derrien J, Alberge JB, Douillard E, Devic M, Deshayes S, Campion L, Westermann F, Moreau P, Herrmann C, Bourdon J, Magrangeas F, Minvielle S. Exploring the impact of dexamethasone on gene regulation in myeloma cells. Life Sci Alliance. 2023 ;6(9):e202302195. doi: 10.26508/lsa.202302195.
- Stockhammer P, Baumeister H, Ploenes T, Bonella F, Theegarten D, Dome B, Pirker C, Berger W, Hegedüs L, Baranyi M, Schuler M, Deshayes S, Bölükbas S, Aigner C, Blanquart C, Hegedüs B. Krebs von den Lungen 6 (KL-6) is a novel diagnostic and prognostic biomarker in pleural mesothelioma. Lung Cancer. 2023. 185:107360 https://doi.org/10.1016/j.lungcan.2023.107360.
- Labbé M, Menoret E, Letourneur F, Saint-Pierre B, de Beaurepaire L, Veziers J, Dreno B, Denis MG, Blanquart C, Boisgerault N, Fonteneau JF, Fradin D. TP53 mutations correlate with the non-coding RNA content of small extracellular vesicles in melanoma. Journal of Extracellular Biology. 2023. Volume 2, Issue 8. 2:e105.
- Boisgerault N, Bertrand P. Inside PD-1/PD-L1,2 with their inhibitors. Eur J Med Chem. 2023 Aug 5;256:115465. doi: 10.1016/j.ejmech.2023.115465. Epub 2023 May 6. PMID: 37196547.
- ChemR23 activation reprograms macrophages toward a less inflammatory phenotype and dampens carcinoma progression. Front Immunol. 2023 Jul 19;14:1196731. doi: 10.3389/fimmu.2023.1196731. eCollection 2023.
- Grard M, Idjellidaine M, Arbabian A, Chatelain C, Berland L, Combredet C, Dutoit S, Deshayes S, Dehame V, Labarrière N, Fradin D, Boisgerault N, Blanquart C, Tangy F, Fonteneau JF. Oncolytic attenuated measles virus encoding NY-ESO-1 induces HLA I and II presentation of this tumor antigen by melanoma and dendritic cells. Cancer Immunol Immunother. 2023 Jul 19. doi: 10.1007/s00262-023-03486-4. Online ahead of print.PMID: 37466668.
- Fradin D, Tost J, Busato F, Mille C, Lachaux F, Deleuze JF, Apter G, Benachi A. DNA methylation dynamics during pregnancy. Front Cell Dev Biol. 2023 May 22;11:1185311. doi: 10.3389/fcell.2023.1185311. eCollection 2023.PMID: 37287456.
- Bourreau C, Treps L, Faure S, Fradin D, Clere N. Therapeutic strategies for non-small cell lung cancer: Experimental models and emerging biomarkers to monitor drug efficacies. Pharmacol Ther. 2023 Jan 12:108347. doi: 10.1016/j.pharmthera.2023.108347.
- Sun S, Qi W, Rehrauer H, Ronner M, Hariharan A, Wipplinger M, Meiller C, Stahel R, Früh M, Cerciello F, Fonteneau JF, Jean D, Felley-Bosco E. Viral Mimicry Response Is Associated With Clinical Outcome in Pleural Mesothelioma. JTO Clin Res Rep. 2022 Nov 7;3(12):100430.
- Paternot S, Raspé E, Meiller C, Tarabichi M, Assié JB, Libert F, Remmelink M, Bisteau X, Pauwels P, Blum Y, Le Stang N, Tabone-Eglinger S, Galateau-Sallé F, Blanquart C, Van Meerbeeck JP, Berghmans T, Jean D, Roger PP. Preclinical evaluation of CDK4 phosphorylation predicts high sensitivity of pleural mesotheliomas to CDK4/6 inhibition. Mol Oncol. 2022 Nov 30. doi: 10.1002/1878-0261.13351.
- Hariharan A, Qi W, Rehrauer H, Wu L, Ronner M, Wipplinger M, Kresoja-Rakic J, Sun S, Oton-Gonzalez L, Sculco M, Serre-Beinier V, Meiller C, Blanquart C, Fonteneau JF, Vrugt B, Rüschoff JH, Opitz I, Jean D, de Perrot M, Felley-Bosco E. Heterogeneous RNA editing and influence of ADAR2 on mesothelioma chemoresistance and the tumor microenvironment. Mol Oncol. 2022 Oct 11. doi: 10.1002/1878-0261.13322.
- Mai HL, Deshayes S, Nguyen TV, Dehame V, Chéné AL, Brouard S, Blanquart C. IL-7 is expressed in malignant mesothelioma and has a prognostic value. Mol Oncol. 2022 Aug 28. doi: 10.1002/1878-0261.13310.
- Autin P, Deshayes S, Lea J, Boisgerault N, Dupré E, Labarrière N, Leguevel R, Fonteneau JF, Blanquart C, Fradin D.The DCMU Herbicide Shapes T-cell Functions By Modulating Micro-RNA Expression Profiles. Front Immunol. 2022 Jul 28;13:925241. doi: 10.3389/fimmu.2022.925241. eCollection 2022.
- Treps L, Ager A, Hida K. Editorial: Tumor Vessels as Directors of the Tumor Microenvironment: New Findings, Current Challenges & Perspectives. Front Cell Dev Biol. 2022 Mar 29;10:885670. doi: 10.3389/fcell.2022.885670. eCollection 2022.
- Chaumette T, Cinotti R, Mollé A, Solomon P, Castain L, Fourgeux C, McWilliam HE, Misme-Aucouturier B, Broquet A, Jacqueline C, Vourc'h M, Fradin D, Bossard C, David L, Montassier E, Braudeau C, Josien R, Villadangos JA, Asehnoune K, Bressollette-Bodin C, Poschmann J, Roquilly A. Monocyte Signature Associated with Herpes Simplex Virus Reactivation and Neurological Recovery After Brain Injury. Am J Respir Crit Care Med. 2022 Apr 29.
- Mathiot L, Herbreteau G, Robin S, Fenat C, Bennouna J, Blanquart C, Denis M, Pons-Tostivint E. HRAS Q61L Mutation as a Possible Target for Non-Small Cell Lung Cancer: Case Series and Review of Literature. 2022. Curr Oncol. May 20;29(5):3748-3758.
- Blondy T, Poly J, Linot C, Boucard J, Allard-Vannier E, Nedellec S, Hulin P, Hénoumont C, Larbanoix L, Muller RN, Laurent S, Ishow E and Blanquart C. Impact of RAFT chain transfer agents on the polymeric shell density of magneto-fluorescent nanoparticles and their cellular uptake. 2022. Nanoscale. 14(15):5884-5898.
- Laquinta S, Khazaie S, Ishow É, Blanquart C, Fréour S, Jacquemin F. Influence of the mechanical and geometrical parameters on the cellular uptake of nanoparticles: a stochastic approach. 2022. Int J Numer Method Biomed Eng. Mar 27:e3598. doi: 10.1002/cnm.3598.
- Charrier M, Lorant J, Contreras-Lopez R, Téjédor G, Blanquart C, Lieubeau B, Schleder C, Leroux I, Deshayes S, Fonteneau JF, Babarit C, Hamel A, Magot A, Péréon Y, Viau S, Delorme B, Luz-Crawford P, Lamirault G, Djouad F, Rouger K. Human MuStem cells exert inhibitory function on T-cell proliferation and cytotoxicity through paracrine and contact-dependent pathways. 2022. Stem Cell Res. 10;13(1):7.
- Marazioti A, Krontira AC, Behrend SJ, Giotopoulou GA, Ntaliarda G, Blanquart C, Bayram H, Iliopoulou M, Vreka M, Trassl L, Pepe MAA, Hackl CM, Klotz LV, Weiss SAI, Koch I, Lindner M, Hatz RA, Behr J, Wagner DE, Papadaki H, Antimisiaris SG, Jean D, Deshayes S, Grégoire M, Kayalar Ö, Mortazavi D, Dilege Ş, Tanju S, Erus S, Yavuz Ö, Bulutay P, Fırat P, Psallidas I, Spella M, Giopanou I, Lilis I, Lamort AS, Stathopoulos GT. KRAS signaling in malignant pleural mesothelioma. 2022. EMBO Mol Med. 13:e13631.
- Pons-Tostivint E, Lugat A, Fontenau JF, Denis MG, Bennouna J. STK11/LKB1 Modulation of the Immune Response in Lung Cancer: From Biology to Therapeutic Impact. 2021. Cells. 11;10(11):3129.
- Ollivier L, Labbé M, Fradin D, Potiron V, Supiot S. Interaction Between Modern Radiotherapy and Immunotherapy for Metastatic Prostate Cancer. 2021. Front Oncol. 11:744679.
- Ollivier L, Guimas V, Rio E, Vaugier L, Masson I, Libois V, Labbé M, Fradin D, Potiron V, Supiot S. 2021. Combination radiotherapy-immunotherapy in genitourinary cancer. Cancer Radiother. 25(6-7):565-569.
- Grard M, Chatelain C, Delaunay T, Pons-Tostivint E, Bennouna J, Fonteneau JF. 2021. Homozygous Co-Deletion of Type I Interferons and CDKN2A Genes in Thoracic Cancers: Potential Consequences for Therapy. Front Oncol. 11:695770.
- Lavy M, Gauttier V, Poirier N, Barillé-Nion S, Blanquart C. 2021. Specialized Pro-Resolving Mediators Mitigate Cancer-Related Inflammation: Role of Tumor-Associated Macrophages and Therapeutic Opportunities. Front Immunol. Jun 30;12:702785.
- Briolay T, Petithomme T, Fouet M, Nguyen-Pham N, Blanquart C, Boisgerault N. 2021. Delivery of cancer therapies by synthetic and bio-inspired nanovectors. Mol Cancer. 20(1):55.
- Sun S, Frontini F, Qi W, Hariharan A, Ronner M, Wipplinger M, Blanquart C, Rehrauer H, Fonteneau JF, Felley-Bosco E. 2021. Endogenous retrovirus expression activates type-I interferon signaling in an experimental mouse model of mesothelioma development. Cancer Lett. 10:S0304-3835(21)00108-7.
- Tournier P, Guicheux J, Paré A, Maltezeanu A, Blondy T, Veziers J, Vignes C, André M, Lesoeur J, Barbeito A, Bardonnet R, Blanquart C, Corre P, Geoffroy V, Weiss P, Gaudin A. 2021. A partially demineralized allogeneic bone graft: in vitro osteogenic potential and preclinical evaluation in two different intramembranous bone healing models. Sci Rep. Mar 1;11(1):4907.
- Soamalala J, Diot S, Pellerano M, Blanquart C, Galibert M, Jullian M, Puget K, Morris MC. 2021. Fluorescent Peptide Biosensor for Probing CDK6 Kinase Activity in Lung Cancer Cell Extracts. Chembiochem. 22(6):1065-1071.
- Petithomme T, Grard M, Fonteneau JF, Boisgerault N. Le vaccin contre la fièvre jaune : un nouveau traitement anti-tumoral ? Médecine/Sciences. 2020 Dec;36(12):1216-1217.
- Delaunay T, Nader J, Grard M, Farine I, Hedwig V, Foloppe J, Blondy T, Violland M, Pouliquen D, Grégoire M, Boisgerault N, Erbs P, Fonteneau JF. High Oncolytic Activity of a Double-Deleted Vaccinia Virus Copenhagen Strain against Malignant Pleural Mesothelioma. Molecular Therapy Oncolytics. 2020 Aug 25;18:573-578.
- Vallion R, Divoux J, Glauzy S, Ronin E, Lombardi Y, Lubrano di Ricco M, Grégoire S, Nemazanyy I, Durand A, Fradin D, Lucas B, Salomon BL. Regulatory T Cell Stability and Migration Are Dependent on mTOR. Journal of Immunology 2020 Oct 1;205(7):1799-1809
- Labbé M, Hoey C, Ray J, Potiron V, Supiot S, Liu SK, Fradin D. microRNAs identified in prostate cancer: Correlative studies on response to ionizing radiation. Molecular Cancer 2020 Mar 23;19(1):63
- Parrot T, Oger R, Allard M, Desfrançois J, Raingeard de la Blétière D, Coutolleau A, Preisser L, Khammari A, Dréno B, Delneste Y, Guardiola P, Fradin D*, Gervois N*. *co-dernier auteurs. Transcriptomic features of tumour-infiltrating CD4lowCD8high double positive αβ T cells in melanoma. Scientific Reports 2020 Apr 3;10(1):5900
- Gauttier V, Pengam S, Durand J, Biteau K, Mary C, Morello A, Néel M, Porto G, Teppaz G, Thepenier V, Danger R, Vince N, Wilhelm E, Girault I, Abes R, Ruiz C, Trilleaud C, Ralph K, Trombetta ES, Garcia A, Vignard V, Martinet B, Glémain A, Bruneau S, Haspot F, Dehmani S, Duplouye P, Miyasaka M, Labarrière N, Laplaud D, Le Bas-Bernardet S, Blanquart C, Catros V, Gouraud PA, Archambeaud I, Aublé H, Metairie S, Mosnier JF, Costantini D, Blancho G, Conchon S, Vanhove B, Poirier N. Selective SIRPα blockade reverses tumor T cell exclusion and overcomes cancer immunotherapy resistance. J Clin Invest. 2020. 130(11):6109-6123.
- Kara-Terki L, Treps L, Blanquart C, Fradin D. Critical Roles of Tumor Extracellular Vesicles in the Microenvironment of Thoracic Cancers. Int J Mol Sci. 2020. 21(17):E6024.
- Jean D, Delaunay T, Meiller C, Boisgerault N, Grard M, Caruso S, Blanquart C, Felley-Bosco E, Bennouna J, Tangy F, Grégoire M, Fonteneau JF. Reply to: Oncolytic Viral Therapy for Malignant Pleural Mesothelioma. J Thorac Oncol. 2020. 15(7):e113-e116.
- Blondy T, d’Almeida SM, Briolay T, Tabiasco J, Meiller C, Chéné AL, Cellerin L, Deshayes S, Delneste Y, Fonteneau JF, Boisgerault N, Bennouna J, Grégoire M, Jean D, Blanquart C. Involvement of the M-CSF/IL-34/CSF-1R pathway in malignant pleural mesothelioma. J Immunother Cancer. 2020. 8(1):e000182.
- Blanquart C, Jaurand MC, Jean D. The Biology of Malignant Mesothelioma and the Relevance of Preclinical Models. Front Oncol. 2020. 25;10:388
- Delaunay T, Achard C, Boisgerault N, Grard M, Petithomme T, Chatelain C, Dutoit S, Blanquart C, Royer PJ, Minvielle S, Quetel L, Meiller C, Jean D, Fradin D, Bennouna J, Magnan A, Cellerin L, Tangy F, Grégoire M, Fonteneau JF. Frequent homozygous deletions of type I interferon genes in pleural mesothelioma confer sensitivity to oncolytic measles virus. J Thorac Oncol. 2020. 15(5):827-842.
- Delaunay T, Achard C, Grégoire M, Tangy F, Boisgerault N, Fonteneau JF. A Functional Assay to Determine the Capacity of Oncolytic Viruses to Induce Immunogenic Tumor Cell Death. Methods Mol Biol. 2020. 2058:127-132.
- Vignard V, Labbé M, Marec N, André-Grégoire G, Jouand N, Fonteneau JF, Labarrière N, Fradin D. MicroRNAs in Tumor Exosomes Drive Immune Escape in Melanoma. Cancer Immunol Res. 2020. Feb;8(2):255-267.
- Marotte L, Simon S, Vignard V, Dupré E, Gantier M, Cruard J, Alberge JB, Hussong M, Deleine C, Heslan JM, Schaffer J, Fradin D, Jarry A, N’Guyen T, Labarrière N. Increased anti-tumor efficacy of Human PD-1 deficient Melanoma-specific Lymphocytes. J ImmunoTher Cancer. 2020. 8(1). pii: e000311.
- Vanbervliet-Defrance B, Delaunay T, Daunizeau T, Kepenekian V, Glehen O, Weber K, Estornes Y, Ziverec A, Djemal L, Delphin M, Lantuéjoul S, Passot G, Grégoire M, Micheau O, Blanquart C, Renno T, Fonteneau JF, Lebecque S, Mahtouk K. Cisplatin unleashes Toll-like receptor 3-mediated apoptosis through the downregulation of c-FLIP in malignant mesothelioma. Cancer Letters. 2020. 1;472:29-39.
- Samimi M, Benlalam H, Aumond P, Gaboriaud P, Fradin D, Kervarrec T, Florenceau L, Vignard V, Blom A, Touzé A, Gervois N, Labarriere N. Viral and tumor antigen-specific CD8 T-cell responses in Merkel cell carcinoma. Cell Immunol. 2019. 344:103961.
- Guillaume T, Dehame V, Chevallier P, Peterlin P, Garnier A, Grégoire M, Pichinuk E, Rubinstein DB, Wreschner DH. Targeting cell-bound MUC1 on myelomonocytic, monocytic leukemias and phenotypically defined leukemic stem cells with anti-SEA module antibodies. Exp Hematol. 2019. 70:97-108.
- Bertrand P*, Blanquart C*, Héroguez V*. The ROMP: A Powerful Approach to Synthesize Novel pH-Sensitive Nanoparticles for Tumor Therapy. Biomolecules. 2019. 12;9(2).
- Bouchet S, Linot C, Ruzic D, Agbaba D, Fouchaq B, Roche J, Nikolic K, Blanquart C, Bertrand P. Extending Cross Metathesis To Identify Selective HDAC Inhibitors: Synthesis, Biological Activities, and Modeling. ACS Med Chem Lett. 2019. 10(6):863-868.
- Boucard J, Briolay T, Blondy T, Boujtita M, Nedellec S, Hulin P, Grégoire M, Blanquart C*, Ishow E*. Hybrid Azo-fluorophore Organic Nanoparticles as Emissive Turn-on Probes for Cellular Endocytosis. ACS Appl Mater Interfaces. 2019. 11(36):32808-32814.
- Autin P, Blanquart C, Fradin D. Epigenetic Drugs for Cancer and microRNAs: A Focus on Histone Deacetylase Inhibitors. Cancers (Basel). 2019. 11(10).
- Blanquart C, Linot C, Cartron PF, Tomaselli D, Mai A, Bertrand P. Epigenetic metalloenzymes. Curr Med Chem. 2019. 26(15):2748-2785.





